Daily Business Report/Jan. 10, 2017
Median home prices have bested their pre-recession levels.
December Housing Sales
Finished Out a Strong Year
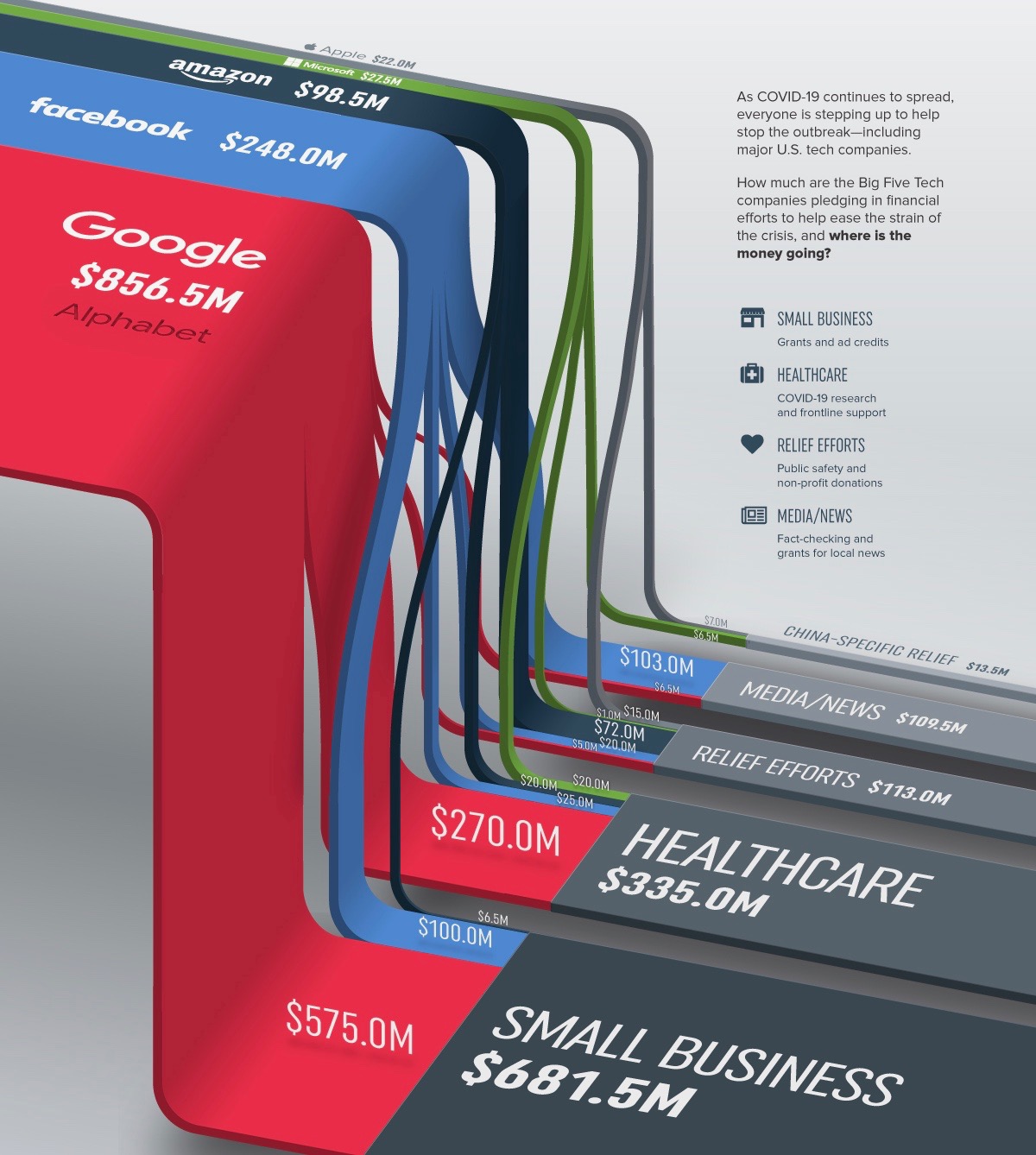
Despite a dramatic low in the inventory of homes for sale, 2016 saw a strong number of transactions and higher home prices in San Diego County, according to housing statistics from the Greater San Diego Association of Realtors.
Single-family home sales were down by 8 percent in December compared to November, but the number of sales for the entire year were virtually the same as for 2015. Attached properties (condominiums and townhomes) also saw a drop in December, down 14 percent from November. However, for the year 2016, condos and townhomes posted a 3 percent increase in sales compared to 2015.
Median home prices have bested their pre-recession levels. Single-family homes prices rose 2 percent in December, month-over-month, and for the entire year were over 6.5 percent higher. Condo and townhome sale prices were up slightly in December, but for the entire year rose by more than 7 percent.
Unfortunately, the supply of resale homes on the market slipped to 1.5 months. (Five to six months is considered a healthy level.) In 2016, the region’s homes were on the market an average of 33 days before close of escrow, compared to 39 days for the year 2015.
“Prospective home buyers and real estate professionals are challenged by low inventory levels and higher competition for those fewer properties,” said SDAR’s President Bob Kevane. “But we are as busy as ever, and opportunities abound for those who are diligent in their home search.”
In December, the ZIP codes in San Diego County with the most single-family home sales were:
- 92064 (Poway) with 46
- 92028 (Fallbrook) with 45
- 91977 (Spring Valley) with 43
- 92009 (Carlsbad Southeast) with 41
- 92027 (Escondido East) with 40
The most expensive property sold in the county last month was a 3,500-square-foot, 5-bedroom, 4-bath oceanfront estate in Del Mar, built in 2004, with a price of $18 million.
________________________
Illumina Unveils New Technology
For Under $100 Genome Sequencing
Illumina launched a new high-throughput sequencing instrument, NovaSeq, at the JP Morgan Healthcare Conference in San Francisco this week, which it will begin shipping in limited quantities by the end of the first quarter.
The platform makes use of nanofabricated flow cells and specially designed optics that CEO Francis deSouza said would in future iterations enable a human genome to be sequenced for $100, although he did not provide a timeline.
“We can see what it would take to get there,” deSouza said. “It will be a lot of work but we feel that every component we have in the system has enough head room that there is a path to get there.”
DeSouza also said that the firm expects fourth quarter revenues to be up 5 percent to $619 million. He provided an update of the company’s Firefly project — a semiconductor-based low-cost sequencing system — and noted that its spinoff Helix planned to have a “major launch” mid year. Also this week, Illumina and Bio-Rad jointly launched a single-cell sequencing workflow, the result of a collaboration between the two firms that began last year.
Illumina plans to launch two configurations of NovaSeq — the NovaSeq 6000, which will cost $985,000 and begin shipping by the end of the quarter, and the NovaSeq 5000, which will cost $850,000 and begin shipping this summer — as well as four different flow cells.
________________________
State of the City Address
Mayor Kevin Faulconer’s annual State of the City address will be Thursday at 6 p.m. in the Balboa Theatre, 868 Fourth Ave., in Downtown San Diego. A reception follows at the US Grant Hotel.
________________________

Surprising At-Risk Group for Skin Cancer
By Gina Jacobs | SDSU NewsCenter
In a new study of indoor tanning and skin cancer risk, the use of indoor tanning among non-heterosexual black male teens was found to be nearly equal to that of heterosexual white females. The study, led by San Diego State University researcher Aaron Blashill, was recently published in the journal JAMA Dermatology.
“Contrary to popular thought, racial and ethnic minorities engage in indoor tanning and it appears to be particularly concentrated among sexual-minority adolescent boys,” said Blashill, an assistant professor of psychology.
The data comes from the 2015 Youth Risk Behavior Survey, a nationally representative survey that examines the prevalence of risky health behaviors among 9th- to 12th-grade public and private school students.
Earlier studies have shown non-heterosexual minority males have one of the highest known prevalence rates of skin cancer, with up to twice the risk of heterosexual males.
Confusion over why people with darker skin might use indoor tanning stems from an incomplete understanding of why people tan in the first place, Blashill said.
“Many only think of indoor tanning as something people do to darken their skin, so the idea that a black individual would tan at all is hard for some to grasp,” he said. “But if we think of indoor tanning as a coping strategy, then the findings begin to make more sense.”
Because UV exposure can induce relaxation through the release of natural opioids in the brain, it’s possible that non-heterosexual black and Hispanic teenage boys engage in indoor tanning to help regulate psychological distress, which could be the result of discrimination, prejudice, and victimization based on their sexual orientation and/or race and ethnicity, Blashill explained.
While indoor tanning is banned for minors in California and several other states, Blashill said these restrictions are not 100 percent effective.
“It is important to understand what’s driving indoor tanning among these boys so we can develop future skin cancer prevention and education campaigns targeted at the high-risk group.”
Blashill is currently working on a follow-up study evaluating the indoor tanning use of 14- to 35-year-old sexual-minority males in San Diego County to see if there is a similar phenomenon among college-aged and young adults.
________________________
SANDAG to Conduct
Transportation Survey
This week SANDAG will launch phase two of the San Diego Regional Transportation Study, inviting approximately 200,000 households in San Diego County to participate in a survey of their travel behavior. Postcard invitations will begin to arrive in mailboxes this week.
The information gathered for the study will help transportation planners better understand how, when, and why residents travel in the region. The results will be used to help develop infrastructure projects and programs to better meet regional transportation needs.
Most participants will be asked to use a smartphone application to answer questions about their daily travel choices, with some respondents completing the study online or by phone.
Participants will be prompted to answer questions about when and where they travel; whether they drive alone, carpool, vanpool, walk, bike, or use public transit; and how much their travel activity costs (e.g., parking and transit fares).
SANDAG has engaged Resource Systems Group Inc. to help conduct this study. For more information, visit sandag.org/study.
________________________
Illumina Strikes Deal With Philips, IBM
To Interpret Cancer Genomic Data
Illumina announced two separate collaborations with Royal Philips and with IBM, to advance the analysis and interpretation of genomic data for cancer.
The company’s strategic collaboration with Philips aims to integrate Illumina’s sequencing systems and Philips’ IntelliSpace Genomics clinical informatics platform and to coordinate marketing and sales of the combined solution.
Genomic data from Illumina’s instruments will be acquired using the BaseSpace Sequence Hub and processed through Philips’ IntelliSpace Genomics solution for oncology, which combines data from multiple sources, such as radiology, immunohistochemistry, digital pathology, medical records, and lab tests. Labs adopting the solution will have access to advanced analytics, deep learning technologies and literature, guidelines, and other evidence in a single view.
In addition, Philips and Illumina plan to collaborate on clinical research with health systems in the US that want to develop precision medicine programs in oncology.
“Until now the ability to use genomic data with the aim of having a precise diagnosis of cancer and improve treatment was mostly for the domain of academic centers,” said Jeroen Tas, CEO of Connected Care and Health Informatics at Philips, in a statement. “Through this collaboration we will unlock the value of genomics for a much wider group of laboratories and care providers to help them advance genomics initiatives at greater speed with the aim to offer precision medicine with better outcomes for their patients.”
“We believe that this collaboration will provide an excellent path for our next-generation sequencing systems to be incorporated into health systems in the US and worldwide,” Francis deSouza, president and CEO of Illumina, added in a statement.
Separately, IBM and Illumina plan to integrate Watson for Genomics and Illumina’s BaseSpace and tumor sequencing process in order to standardize and simplify genomic data interpretation.
As a result, researchers using Illumina’s TruSight Tumor 170 cancer sequencing panel will have access to information to help interpret the variant data. In particular, Watson for Genomics will comb professional guidelines, medical literature, clinical trials compendia, and other sources to provide information for each genomic alteration and to produce a report in a process that will take minutes.
The Watson for Genomics software, which adds data from about 10,000 scientific articles and 100 new clinical trials every month, will be available early this year to support the TruSight Tumor 170 assay.
“To enable precision cancer medicine on a large scale, we need new tools to overcome the data barriers of genomic research,” said John Leite, vice president of oncology at Illumina, in a statement. “With a comprehensive assay of Illumina and the power of Watson, we hope to deliver a rapid turnaround of the genomic alteration results.”
“This partnership lays the groundwork for more systematic study of the impact of genomics in oncology,” said Deborah DiSanzo, general manager of IBM Watson Health, in a statement. “Together we are poised to help researchers realize the potential of precision oncology by expanding access to valuable genome sequencing from Illumina and reliable, standardized genomic interpretation from Watson.”